Su mensaje ha sido enviado.
Procesaremos su solicitud y nos pondremos en contacto con usted lo antes posible.
El formulario se ha enviado correctamente.
Encontrará más información en su buzón.

Seleccionar idioma


Cuando se empieza a buscar el mejor ERP farmacéutico, suelen aparecer marcas como SAP, Odoo u Oracle. ¿Pero, son exactamente las más adecuadas para su empresa? La respuesta a esta pregunta requiere un análisis más profundo que una rápida búsqueda en Google. Y en este artículo, haré todo lo que esté en mi mano para ayudarte a decidir por ti mismo cuál es el mejor.
Ahora está claro que el industria farmacéutica necesita algo más que ERP genéricos. Requiere plataformas especializadas que puedan navegar por el laberinto normativo que rige cada proceso. Ahora que el desarrollo de medicamentos cuesta una media de $2.600 millones y los plazos se extienden más de una década, las empresas farmacéuticas no pueden permitir que los ERP les frenen. Gracias a su amplia experiencia en el sector farmacéutico, nuestro equipo de Innowise sabe que cómo elegir un sistema ERP para su negocio, así que siga leyendo para saber más.
Los sistemas ERP optimizan el inventario, garantizan el cumplimiento, mejoran el control de calidad e impulsan decisiones más inteligentes, entre otras cosas. Hay un momento en el que todas las empresas farmacéuticas se encuentran: cuando sus sistemas heredados y herramientas independientes empiezan a causar más problemas de los que resuelven. Esto es lo que ERP arregla cuando se hace bien.
Materias primas y existencias sensibles a la temperatura pueden erosionar silenciosamente los márgenes de las empresas farmacéuticas. Yo se lo digo a los clientes: si su equipo consulta tres sistemas distintos para responder a una comprobación de existencias, es hora de un ERP.
No todos los ERP que dicen estar "preparados para el sector farmacéutico" pueden hacer frente a la realidad cotidiana de la producción regulada, las auditorías estrictas y las cadenas de suministro complejas. Así que dejé a un lado los lustrosos anuncios publicitarios y me centré en lo que más importa a las empresas farmacéuticas.
SAP S/4HANA es el ERP al que recurren algunos de los nombres más importantes del sector farmacéutico, como Pfizer, Merck, Novartis, Johnson & Johnson, AstraZeneca y GlaxoSmithKline. Dispone de módulos específicos diseñados para operaciones de gran envergadura y muy reguladas que requieren procesamiento en tiempo real, cumplimiento estricto de las normativas y control total de los flujos de trabajo de fabricación y cadena de suministro. No es ligero ni rápido de implantar, pero está diseñado para gestionar la escala y la complejidad en múltiples centros y mercados. Las empresas más pequeñas eligen SAP Business One, otro producto de la cartera de SAP.
Los precios de SAP se revelan bajo demanda. En general, sus planes de suscripción se personalizan en función del número de usuarios, los módulos seleccionados, el tipo de implantación (en la nube o in situ) y el nivel de asistencia.
Empresas farmacéuticas medianas y grandes con operaciones en varios países, líneas de producción complejas y requisitos normativos estrictos.
Los precios de Business Central empiezan en $70 y $100 por usuario/mes (pago anual), además ofrecen licencias de $8 por usuario/mes para aquellos con acceso a funcionalidades limitadas (visualización, edición de datos seleccionados, aprobación de flujos).
Empresas farmacéuticas en crecimiento que necesitan funciones ERP básicas con gastos generales gestionables e integración en el entorno Microsoft.
El plan de precios estándar de Odoo con acceso a todos los módulos en modo en línea cuesta €11.90 por usuario/mes durante el primer año de suscripción y €14.90 por usuario/mes en los años siguientes. El plan personalizado que admite implementación on-premise cuesta respectivamente €17.90 y €22.40.
Empresas farmacéuticas que necesitan una solución ERP flexible y escalable, pero están dispuestas a invertir en personalización para cumplir las normas específicas del sector.
Oracle proporciona una estimación de costes individuales bajo demanda. Al igual que otros proveedores de ERP, los precios de NetSuite se basan en suscripciones y se calculan específicamente en función de los módulos que necesite.
Empresas medianas y grandes de los sectores farmacéutico y de ciencias de la vida que necesitan una solución integral con cumplimiento normativo integrado.
Infor CloudSuite utiliza un modelo basado en suscripciones, con precios basados en el número de usuarios y los módulos seleccionados, que se amplían a medida que crecen las empresas. El proveedor comparte los precios bajo demanda.
Empresas farmacéuticas que buscan un ERP basado en la nube con una sólida gestión de la cadena de suministro, funciones de I+D y apoyo al cumplimiento normativo.
Slingshot Pharma es uno de esos ERP que muestra su enfoque láser en las operaciones farmacéuticas, especialmente en torno al cumplimiento y la gestión de la cadena de suministro. Lo que más me ha llamado la atención es su gran compatibilidad con las normativas 21 CFR Parte 11 y GMP. La plataforma ofrece sólidas herramientas de gestión de inventarios y de la cadena de suministro, incluidos almacenes virtuales y modelización de la cadena de suministro, que resultan muy valiosas para las empresas que gestionan complejas redes de proveedores y distribuidores. Con Slingshot, las empresas farmacéuticas pueden obtener información en tiempo real sobre sus operaciones y estar al tanto del cumplimiento de la normativa sin preocuparse constantemente por las lagunas.
Slingshot Pharma ofrece un modelo de precios basado en suscripciones, con costes basados en el número de usuarios y la escala de módulos implementados. Para obtener un presupuesto a medida, lo mejor es consultar a Slingshot o a un socio certificado.
Empresas farmacéuticas y biotecnológicas centradas en ensayos clínicos y desarrollo de productos. Especialmente las que buscan un sistema ERP que pueda gestionar los rigurosos requisitos de cumplimiento y coordinar los esfuerzos de los equipos de investigación y fabricación.
Epicor Kinetic emplea un modelo de precios basado en suscripción y comparte los precios exactos bajo demanda. Las implementaciones en las instalaciones y los módulos adicionales pueden incurrir en cargos por separado.
Fabricantes farmacéuticos que buscan una solución ERP escalable que equilibre el cumplimiento de la normativa con la eficiencia operativa, especialmente aquellos con procesos de producción más complejos.
Sage X3 me llamó la atención porque es uno de los productos más maduros que se ha ido perfeccionando a lo largo de las décadas para afrontar los retos específicos del sector farmacéutico. Lo que lo distingue es que Sage X3 ofrece funciones específicas del sector con la flexibilidad necesaria para adaptarse a requisitos empresariales concretos, ya que admite tanto la fabricación por procesos para productos farmacéuticos como la fabricación discreta para dispositivos médicos. La plataforma cumple todos los requisitos y puede ayudar a las empresas farmacéuticas a estar preparadas para las auditorías de procesos y productos sin complicaciones.
Sage X3 ofrece costes a medida y sigue un modelo tradicional de licencias locales con precios basados en el usuario, aunque existen opciones de implantación en la nube con alternativas de suscripción.
Compañías farmacéuticas de tamaño medio-grande que necesitan capacidades de cumplimiento demostradas y tienen requisitos de fabricación complejos.
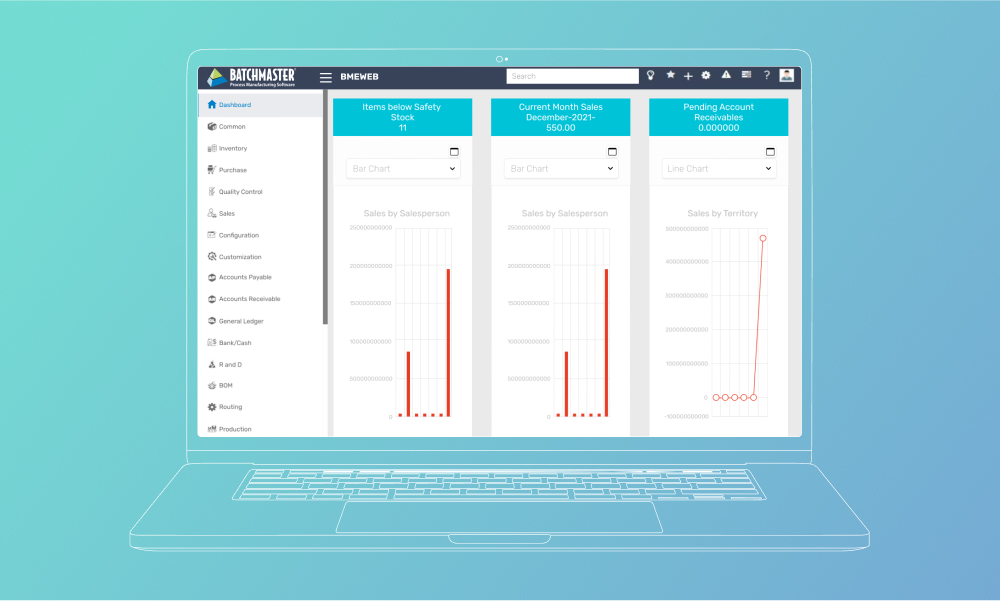
BatchMaster revela los costes previa solicitud y sigue un enfoque de precios modular con costes que varían en función del número de usuarios y los módulos de fabricación seleccionados, disponibles tanto en las instalaciones como en la nube.
Pequeños y medianos fabricantes farmacéuticos que necesitan sólidas capacidades de fabricación por lotes pero desean conservar sus sistemas financieros existentes y evitar revisiones masivas de ERP.
SYSPRO es uno de esos ERP que no intenta serlo todo para todos. En su lugar, se centra en la fabricación farmacéutica realmente bien. Me gusta cómo han desarrollado funcionalidades específicas para el sector farmacéutico sin abandonar su principal fortaleza en las operaciones de fabricación. SYSPRO permite a los equipos gestionar eficazmente el control de calidad, las compras, la gestión de inventarios, las operaciones de taller, las ventas y las finanzas desde un único sistema integrado, diseñado específicamente para las necesidades de la industria.
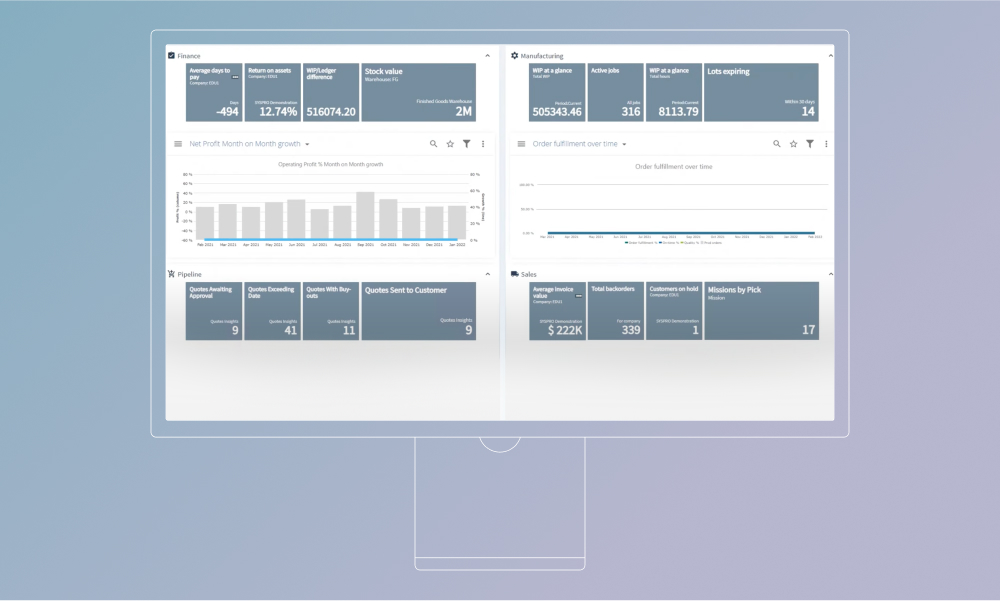
Póngase en contacto directamente con SYSPRO para conocer los precios que se ajustan exactamente a sus necesidades. En general, la empresa sigue un modelo de precios tradicional basado en licencias, en el que el número de usuarios y la selección de módulos determinan los costes.
Fabricantes farmacéuticos de tamaño medio que dan prioridad a operaciones de fabricación sólidas y necesitan funciones de cumplimiento fiables sin la complejidad de los sistemas de nivel empresarial más grandes.
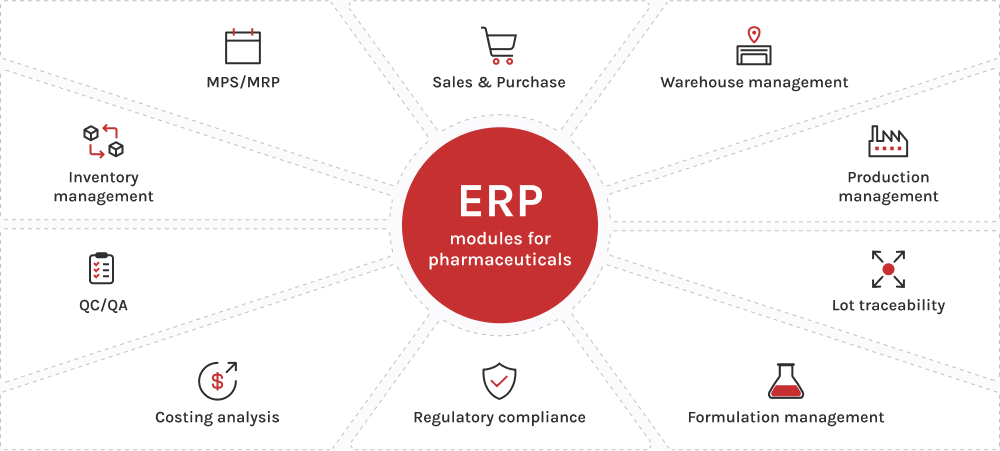
Recapitulemos los módulos ERP esenciales para la industria farmacéutica que mantienen a las empresas en lo más alto de su sector.
















Las empresas farmacéuticas están sometidas a una presión increíble para crear productos innovadores y, al mismo tiempo, hacer frente a normativas en constante cambio. Por eso es esencial contar con el sistema ERP adecuado. Trabajamos en estrecha colaboración con las empresas farmacéuticas para diseñar soluciones que les ayuden a ofrecer productos a los pacientes con mayor rapidez. Se trata de hacer un uso inteligente de los datos para tomar mejores decisiones en todos los ámbitos.

Gestor de cartera de tecnologías médicas y sanitarias
Hemos realizado implantaciones, personalizaciones, migraciones e integraciones de ERP tanto para empresas farmacéuticas como para empresas de fabricación de genéricos.










Su mensaje ha sido enviado.
Procesaremos su solicitud y nos pondremos en contacto con usted lo antes posible.

Al registrarse, acepta nuestra Política de privacidadincluyendo el uso de cookies y la transferencia de su información personal.